‘Ghost heart’: Made from scaffolds of pig and patient cells, this cardiac breakthrough could soon be ready for transplant in humans
“It really changed my life,” said Taylor, who until 2020 directed regenerative medicine research at the Texas Heart Institute in Houston. “I said to myself, ‘Oh my God, this is life.’ I wanted to find out how and why, and how to recreate it to save lives.”
That goal has become a reality. At the Life Itself conference on Wednesday, a health and wellness program presented in partnership with CNN, Taylor showed viewers the scaffolding of a pig’s heart infused with human stem cells—making a viable, human heartbeat the body would not reject. Why? Because it is made of that person’s own tissues.
“Now we can really imagine building a personalized human heart, from an emergency procedure to a heart transplant where you’re so sick, to a planned procedure,” Taylor told the audience.
“It (antirejection) lowers your risk by eliminating the need for drugs, it lowers costs by using your own cells to make that heart … and you’re not in the hospital as often so it’s your heart.” Improves quality of life,” she said.
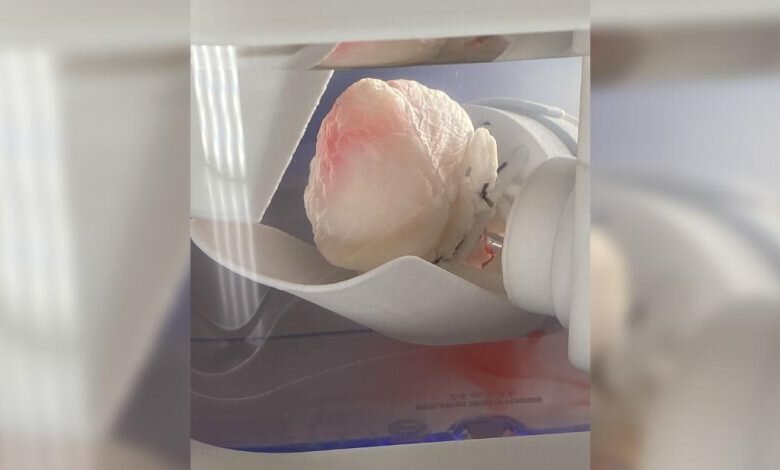
beginning On stage with them was BAB, a robotic teller taught to inject stem cells into the chambers of ghost hearts inside a sterile environment. As seen by viewers of Life Hi Working in a sterile environment, Taylor shows video of a pearly white mass called a “ghost heart” that begins to turn pink.
“This is the first shot to truly cure the number one killer of men, women and children around the world – heart disease. And then I want to make it available to everyone,” Taylor said to the applause of the audience.
“He never gave up,” said Michael Galway, lead inventor of BAB and president and CEO of Advanced Solutions, which designs and builds platforms for manufacturing human tissues.
“At any point, Dr. Taylor could have simply said ‘I’m done, this won’t work. But she persisted for years, fighting setbacks to find the right types of cells in the right amounts and under the right conditions’ . Enable those cells to be happy and grow.”
give birth to heart
Taylor’s fascination with growing hearts began in 1998, when she was part of a team at Duke University that injected cells into a rabbit’s failing heart, creating new heart muscle. As trials began in humans, however, the process was hit or miss.
“We were putting cells into damaged or scarred areas of the heart and hoping that would reverse the existing damage,” she told CNN. “I started thinking: What if we could get rid of that bad environment and rebuild the house?”
Taylor’s first breakthrough came in 2008 when he and a team from the University of Minnesota washed cells from rat hearts and began working with the translucent skeleton left behind.
Soon, he graduated to using pig hearts, due to their physical resemblance to the human heart.
“We took a pig’s heart, and we washed all the cells with a gentle baby shampoo,” she said. “What was left was an extracellular matrix, a transparent structure we called the ‘ghost heart.
“Then we infected the blood vessel cells and let them grow on the matrix for a couple of weeks,” Taylor said. “It created a way to feed the cells we were going to add as we rewired the blood vessels in the heart.”
The next step was to start injecting immature stem cells into different areas of the scaffold, “and then we had to teach the cells to grow.”
Taylor said, “We should stimulate them electrically like a pacemaker, but very slowly at first, until they get stronger and stronger. First, cells in one place will twitch, then cells in another place will twitch.” Will bite, but they’re not together,” Taylor said. , “Over time they start to connect to each other in the Matrix and by about a month, they start beating together like hearts. And let me tell you, it’s a ‘wow’ moment!”
But that’s not the end of “mothering” Taylor and her team. Now he must nurture the budding heart by giving it blood pressure and teaching it to pump.
“We fill the heart chambers with artificial blood and let the heart cells squeeze against it. But we must help them with electric pumps, or they will die,” she explained.
The cells also get oxygen from the artificial lungs. Taylor said that in the early days all these steps had to be monitored and coordinated 24 hours a day, 7 days a week.
“Hearts have to be eaten every day, and until we build the pieces that make it possible to monitor hearts electronically, someone has to do it personally – and it doesn’t matter that much.” It’s Christmas or New Year’s Day or it’s your birthday,” she said. “It has taken extraordinary groups of people working with me over the years to make this happen.”
But once Taylor and her team saw the consequences of her upbringing, any sacrifices she made became insignificant, “because then there is beauty, the magic,” she said.
“We injected the same types of cells everywhere in the heart, so they all started out the same,” Taylor said. “But now when we look into the left ventricle, we find the left ventricle heart cells. If we look into the atrium, they look like atrial heart cells, and if we look into the right ventricle, they The right ventricle is the cells of the heart.” Told.
“So over time they have evolved depending on where they find themselves and have grown up working together and being hearts. Nature is wonderful, isn’t it?”
billions and billions of stem cells
As her creation came to life, Taylor began dreaming about a day when her prototypical hearts could be mass-produced for the thousands on the transplant list, many of whom die while waiting. . But how do you measure a heart?
“I realized that for every gram of heart tissue we made, we needed a billion heart cells,” Taylor said. “This means we would need 400 billion individual cells for an adult-sized human heart. Now, most laboratories work with a million or more cells, and heart cells don’t divide, which leaves us in dilemma. leaves: where do these cells go?”
The answer came when Japanese biomedical researcher Dr. Shinya Yamanaka discovered that human adult skin cells can be reprogrammed to behave like embryonic or “pluripotent” stem cells, capable of developing into any cell in the body. Huh. The 2007 discovery won the scientist a Nobel Prize, and his “induced pluripotent stem cells (iPS),” soon became known as “Yamanaka factor”.
“Now for the first time we can take blood, bone marrow or skin from a person and grow cells from that person that can turn into heart cells,” Taylor said. “But the scale was still huge: We needed tens of billions of cells. It took us another 10 years to develop the techniques to do this.”
Solution? A honeycomb-like honeycomb of fiber, containing thousands of microscopic pores where cells can attach and be nourished.
“Fiber absorbs nutrients like a coffee filter, the cells have access to the food around them and this allows them to grow in great numbers. We can grow from about 50 million cells to a billion cells a week. Can go up,” Taylor said. “But we need 40 billion or 50 billion or 100 billion, so part of our science over the years has been increasing the number of cells we can grow.”
Another issue: Each heart requires a pristine environment free of contaminants for each step of the process. Every time an intervention had to be made, he and his team risked opening the heart to infection—and death.
“Do you know how long it takes to inject 350 billion cells by hand?” Taylor asked the audience for Life itself. “What if you touch something? You have corrupted the whole heart.”
Once there was an electrical failure in his lab and everyone’s heart died. Taylor and his team were almost inconsolable.
“When something happens to one of these hearts, it’s devastating for all of us,” Taylor said. “And it sounds weird from a scientist, but I had to learn to strengthen my heart emotionally, mentally, spiritually, and physically to go through this process.”
Enter BAB, acronym for BioAssemblyBot, and an “uber-sterile” cradle created by Advance Solutions that can hold the heart and transport it between each step of the process while preserving a germ-free environment. Taylor has now taught BAB the specific process of injecting cells she has painstakingly developed over the past decade.
Galway, the manufacturer of the BAB, said, “When Dr. Taylor is injecting cells, it takes him many years to figure out where to inject, how much pressure to put on the syringe, and when to attach the cells. Good pace and speed.”
“A robot can do this quickly and accurately. And as we know, no two hearts are alike, so BAB can use ultrasound to look inside the vascular pathway of that specific heart, where Dr. Taylor is working blind, so to speak,” Golway said. “It’s exhilarating to watch – at times the hair on the back of my neck really stands out.”
Taylor dropped out of academia in 2020 and is currently working with private investors to take her creations to the masses. If the transplant is successful in humans in upcoming clinical trials, Taylor’s personalized hybrid hearts could be used to save thousands of lives around the world.
In the US alone, about 3,500 people were on the heart transplant waiting list in 2021.
“That’s not counting the people who never make it on the list because of their age or heath,” Taylor said. “If you’re a younger woman, if you’re a minority, if you’re a child, your chances of getting an organ that matches your body are slim.
If you get a heart, within a decade many people get sick or lose their new heart. We can reduce costs, we can increase access, and we can reduce side effects. It’s a win-win.”
Teller can even imagine a day when people bank their own stem cells at a young age, when they need to grow a heart – and one day even a lung, of liver or kidney.
“Say there’s heart disease in their family,” she said. “We can plan ahead: grow their cells to the numbers we need and freeze them, then pull a scaffold off the shelf when they’re diagnosed with heart failure and within two months of heart failure.” build out.
“I am humbled and privileged to be doing this work, and proud of where we are,” she said. “The technology is ready. I hope everyone is with us along for the ride because it’s game-changing.”
Source